Manufacturing Carbonated Water
Although
bottle filling machinery and bottle stoppers evolved considerably during
the second half of the nineteenth century, the process of manufacturing
carbonated water changed very little.
Two methods of carbonated water production evolved, known as the
“English Continuous System,” and the “Standard American System.”
The basic difference between these two approaches was the way in
which the water was carbonated.
The English system enabled bottlers to fill bottles indefinitely,
stopping only to recharge the acid and marble dust in the generator.
Water was drawn continuously into the generator with only
occasional adjustments to pressure valves.
The American system required refilling of the water cylinders
when they were empty. If a
cylinder was dry, water couldn’t be added without wasting the remaining
carbon dioxide it contained.
Although the American system required additional maintenance, it
was the predominant approach used by
In order to bottle with the
American system, the cylinder was filled three-fourths full of water.
Then several pounds of marble dust (calcium carbonate) and
bicarbonate of soda (to keep the marble dust dissolved evenly, soften
the mass, give it a smooth, lump-free texture, and cause the gas to be
more freely generated) were introduced into the marble chamber, along
with enough water to aid in agitation of the mass.
Next, a quantity of sulfuric acid was poured into the acid
chamber and then released into the marble chamber by means of the acid
valve. As the agitator
wheel was turned slowly, the gas was released from the marble dust and
flowed thru the gas pipe and into the cylinder.
This gas dissolved in the water producing carbonated water.
James W. Tufts’ 1888 book,
The Manufacturing and
Bottling of Carbonated Beverages,
listed “Marble-Dust. Price
per barrel, delivered free on board in
As the gas supply decreased, more acid could be let
into the marble chamber to react with any remaining marble dust.
Once the water in the cylinder was charged to the desired
pressure, the discharge valve was opened and the carbonated water
allowed to flow thru a hose to the bottling table.
Due to the corrosive nature of sulfuric acid, generators were
always rinsed thoroughly with fresh water.
Generators were typically six to seven feet tall and
weighed 1,000 pounds, with larger ones weighing as much as four tons.
They were easily kept in working order, but when damaged often
required shipment to the manufacturer for repairs.
Most damage involved collapse of the block tin or lead lining due
to a sudden reduction in pressure.
Foreign objects in the marble dust also tore the lining or bent
agitator blades. The
greatest operating danger posed by generators typically occurred when
the wrong valve was accidentally opened or closed, causing the internal
pressure to rise, exploding the generator and spraying the acidic
contents.
The safe and successful operation of carbonated water
generators required a thorough understanding of how this sophisticated
machinery functioned. For
those seeking additional details, James W. Tufts’ 1888 book,
The Manufacture and
Bottling of Carbonated Beverages,
provides highly detailed descriptions and operating instructions for
setups of one generator with one, two, or three cylinders, and several
other configurations. The
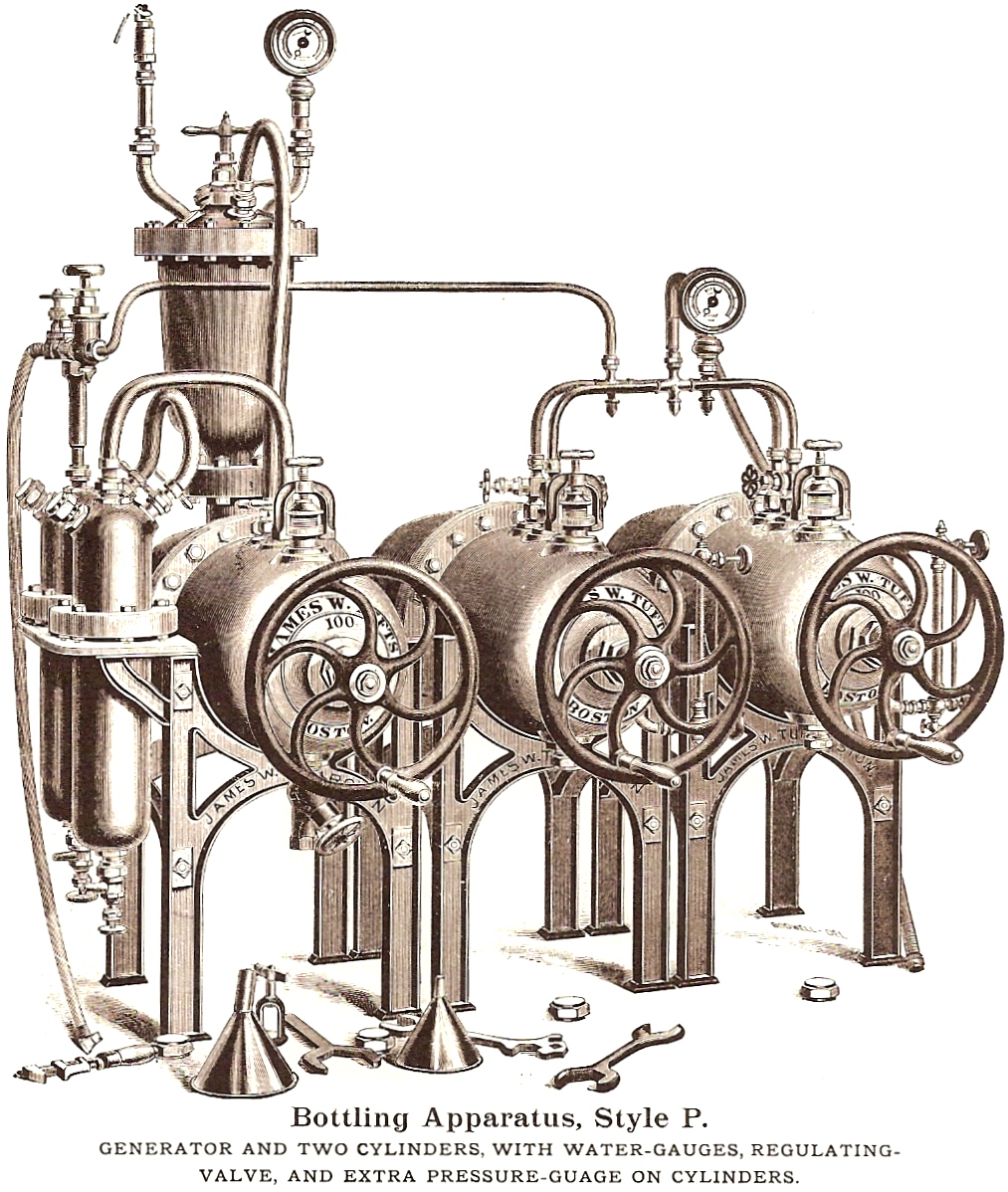
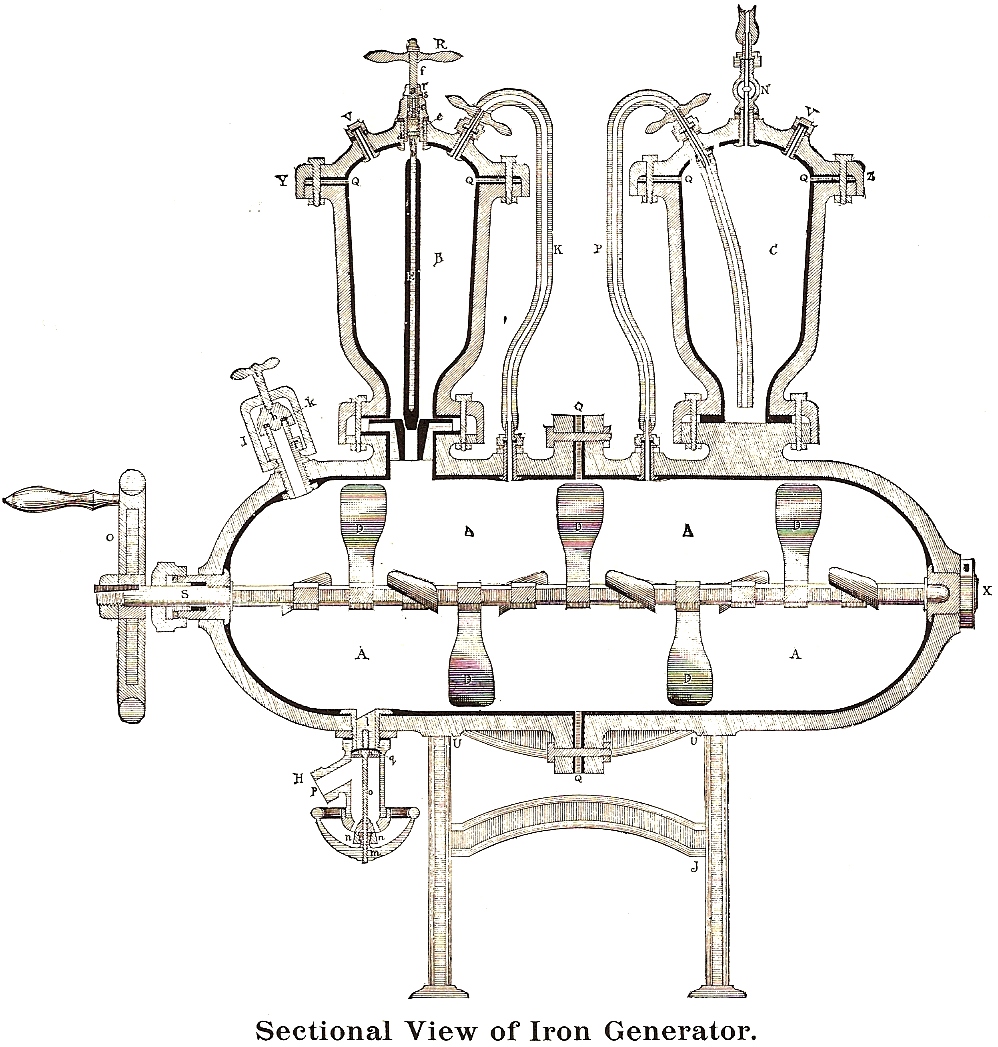
accompanying illustrations show a typical installation (note the tools)
and a sectional view revealing the inner workings of the cylinder, and
acid-chamber, and a purifier.
Tufts produced cylinders of both iron and copper, with both
styles having block tin linings.
 HutchBook.com
HutchBook.com